Exploring the Role of HCOOCH CH2 H2O in Water Interactions and Solutions
Introduction to HCOOCH CH2 H2O
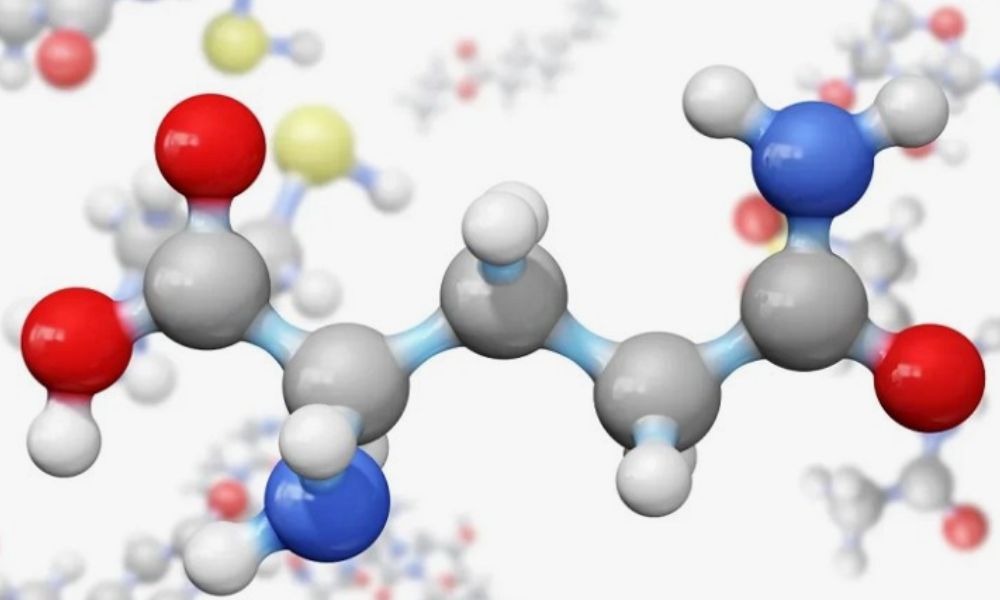
Imagine a molecule that plays a pivotal role in how substances interact with water. HCOOCH CH2 H2O, also known as formate, is more than just a simple compound—it’s a fascinating player in the vast world of chemistry. With its unique structure and properties, it shapes solutions and influences various applications across industries. As we dive deeper into the science behind HCOOCH CH2 H2O, we’ll uncover its impact on water interactions and explore why understanding this relationship is crucial for both research and practical uses. Buckle up as we embark on an intriguing journey into the chemistry of HCOOCH CH2 H2O and its significance in our everyday lives!
The Importance of Water Interactions in Solutions
Water interactions play a crucial role in the behavior of solutions. They influence solubility, reactivity, and stability. Understanding these interactions is essential for various fields, from chemistry to biology.
In solvents like water, molecules exhibit unique behaviors due to hydrogen bonding. This affects how substances dissolve and interact at the molecular level. The polarity of water creates an environment where ionic and polar compounds can thrive or struggle.
Moreover, temperature shifts alter these dynamics significantly. Higher temperatures may increase kinetic energy among molecules, enhancing interaction rates. Conversely, cooler conditions can lead to reduced activity.
The implications stretch beyond theoretical studies; they impact real-world applications in pharmaceuticals and environmental science too. Knowledge of water interactions helps scientists formulate better drugs or design effective cleaning agents that rely on aqueous environments for optimal performance.
How HCOOCH CH2 H2O Affects Water Interactions
HCOOCH CH2 H2O, also known as formyl methanol or glycolic acid methyl ester, plays a significant role in altering water interactions. When introduced into aqueous solutions, its molecular structure allows it to influence hydrogen bonding.
This compound can enhance solubility for various substances. Its presence modifies the arrangement of water molecules around solutes, leading to more effective dissolution processes.
Moreover, HCOOCH CH2 H2O interacts favorably with polar and nonpolar compounds alike. This characteristic enables a broader range of applications in chemical formulations.
The dynamics between HCOOCH CH2 H2O and water are essential in determining reaction rates too. By facilitating enhanced molecular mobility within the solution, HCOOCH2 helps accelerate these reactions under certain conditions.
Its effect on surface tension is noteworthy as well. Lowering this property can improve wetting characteristics which benefits several industrial applications that rely on effective mixing and interaction with liquids.
Applications and Uses of HCOOCH CH2 H2O in Solutions
HCOOCH CH2 H2O, or formyl methanol, finds its way into various applications due to its unique properties. In the field of pharmaceuticals, it acts as a crucial intermediate in drug synthesis. Its ability to interact favorably with water makes it an important component in many formulations.
The compound is also utilized in agrochemicals. It enhances the solubility and effectiveness of active ingredients within pesticide solutions. This results in better absorption by plants and improved pest control.
Moreover, HCOOCH2 plays a role in industrial processes. It serves as a solvent that aids chemical reactions under controlled conditions. The compound’s compatibility with water ensures efficient mixing and improved reaction rates.
In research labs, scientists utilize HCOOCH2 for analytical purposes as well. Its distinct interaction with water allows for precise measurements during experiments involving solution chemistry.
Potential Drawbacks and Risks of Using HCOOCH CH2 H2O in Solutions
While HCOOCH CH2 H2O shows promising benefits in various applications, it’s essential to consider potential drawbacks.
One concern revolves around its solubility and stability in certain conditions. In high concentrations or extreme temperatures, HCOOCH CH2 H2O may degrade, leading to unpredictable results.
Additionally, some studies suggest that prolonged exposure could impact aquatic life. The interaction of this compound with water can produce byproducts harmful to the ecosystem.
There are also regulatory considerations. As research progresses, stricter guidelines may emerge regarding its use in commercial products.
User safety cannot be overlooked. Proper handling is crucial since ingestion or skin contact might pose health risks. Awareness of these factors ensures responsible usage and informed decision-making when incorporating HCOOCH2 into solutions.
Current Research and Future Developments
Current research on HCOOCH2 is uncovering intriguing insights into its interactions with water. Scientists are delving deeper into how this compound influences solubility and stability in various solutions.
Emerging studies focus on its potential role in enhancing the efficacy of pharmaceuticals. By optimizing formulations, researchers aim to improve drug delivery systems that rely on precise water interactions.
Innovations in analytical techniques are also shedding light on molecular behaviors at the microscopic level. These advancements pave the way for tailored applications across industries, from agriculture to biotechnology.
As interest grows, collaborations between universities and industry leaders are expected to drive breakthroughs. The ongoing exploration highlights not only immediate benefits but also long-term implications for environmental sustainability and health sciences.
With every new finding, the future of HCOOCH CH2 H2O promises exciting possibilities that could revolutionize our understanding of chemical interactions in solutions.
Conclusion
HCOOCH CH2 H2O, or formic acid ethyl ester, plays a significant role in the chemistry of water interactions. Its unique molecular structure enables it to influence how substances dissolve and interact with water, making it essential in various applications.
Understanding HCOOCH CH2 H2O impact on water solutions opens up new avenues for research and innovation. It has practical uses across multiple industries, from pharmaceuticals to agriculture. However, as exciting as its potential is, awareness of the possible drawbacks and risks is equally crucial.
Current scientific inquiries continue to explore HCOOCH2 multifaceted properties and their implications for future developments. As researchers delve deeper into this compound’s behavior in aqueous environments, we can anticipate advancements that may further enhance its utility.
The ongoing exploration of HCOOCH2 emphasizes not only its significance but also our responsibility to understand both its benefits and limitations fully. Whether you’re involved in research or simply interested in chemistry’s wonders, keeping an eye on this compound will be rewarding.
Share this content:








Post Comment